Chemistry 1 to 30 Elements Electronic Configuration
What are electronic configurations?
An electronic configuration, also known as an electronic structure, is the arrangement of electrons at different energy levels surrounding an atomic nucleus. The electronic configuration of a molecule is the distribution of electrons in distinct molecular orbitals. The importance of the molecule cannot be overstated. It is possible to determine the number of electrons in bonding and antibonding molecular orbitals from a molecule's or molecular ion's electronic configuration.
-
The electrical configuration of an element is used to figure out where electrons are located in that element.
-
From the lowest to the highest energy level, electrons are arranged in ascending order.
-
The electrical configuration of an element is largely determined by its atomic number.
-
The electrical configuration of an atom is helpful in determining an element's valency, which aids in determining the element's reactivity.
-
The atomic spectra can also be interpreted using the electrical configuration.
-
Noble gases with totally filled outermost electrons, such as Neon, Argon, and Helium, are the most stable. Noble gases have filled valence shells, which give them their inertness.
-
Copper and chromium have a peculiar electrical structure in which the 3d- orbitals are filled first, rather than the 4s orbitals.
-
In chromium([Ar] 3d5 4s1) the d-orbital, which is filled with single electrons, boosts the atom's stability. Similarly, the d-orbital of Copper [Ar] 3d10 4s1 is totally filled with paired electrons, ensuring the atomic structure's stability.
Also check-
-
NCERT Exemplar Class 11th Chemistry Solutions
-
NCERT Exemplar Class 12th Chemistry Solutions
-
NCERT Exemplar Solutions for All Subjects
The four subshell labels are s, p, d, and f, and the electrical configuration of atoms is represented by a sequence of the label names of each atomic subshell, with the total amount of electrons assigned to that specific subshell expressed in superscript. In each of the subshells s, p, d, and f, the maximum number of electrons allowed is 2, 6, 10, and 14 accordingly. Noble gases, which have entirely filled outermost shells and can be prefixed to the outer shell of the element, can also be used to write the electronic configuration of elements, and the electronic configuration must be noted. The electron configuration of an element describes the distribution of electrons in its atomic orbitals. All electron-containing atomic subshells are placed in a sequence in atomic electron configurations, which follows a standard nomenclature (with the number of electrons they possess written in superscript). For example, sodium's electron configuration is 1s2 2s2 2p6 3s1.
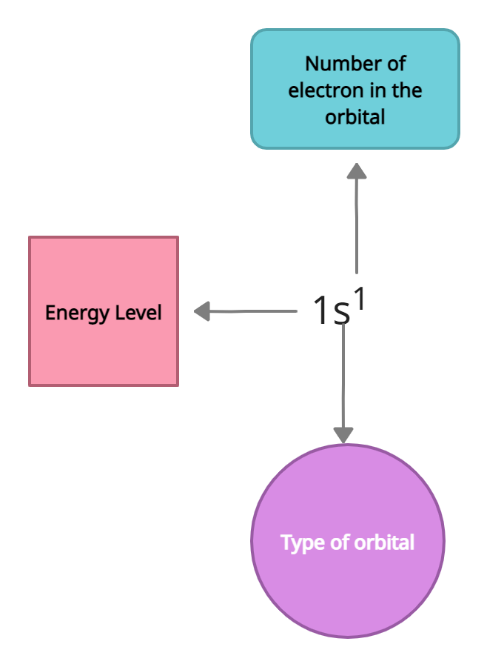
On the other hand, standard notation frequently results in extended electron configurations (especially for elements having a relatively large atomic number). In some cases, a shortened or condensed notation may be used instead of the standard notation. In the abbreviated notation, the series of completely full subshells that correspond to a noble gas's electronic configuration are replaced by the noble gas's symbol in square brackets. As a result, the electron configuration of sodium is [Ne] 3s1 (the electron configuration of neon is 1s2 2s2 2p6, which can be abbreviated to [He] 2s2 2p6).
What are the three rules that must be observed while writing electronic configurations of elements?
-
The Aufbau principle states that before occupying an orbital associated with a higher energy level, electrons must entirely fill the atomic orbitals of the previous energy level. In the sequence of increasing orbital energy level, electrons occupy orbitals.
-
According to Pauli's exclusion principle, No two electrons may have the same values for all four quantum numbers. As a result, each subshell of an orbital may only hold a maximum of two electrons, both of which must have opposite spins.
-
Hund's rule of maximum multiplicity states that all subshells in an orbital must be occupied singly before any subshell can be twice occupied. Furthermore, all electrons in singly occupied subshells must have the same spin (in order to maximise the overall spin).
Electronic configuration of first 30 elements or 1 to 30 elements electronic configuration or Elements 1 to 30
| ATOMIC NUMBER 1 to 30 | ELEMENT | SYMBOL | ELECTRONIC CONFIGURATION | ELECTRON SHELL ARRANGEMENT |
| 1 | HYDROGEN | H | 1s1 | 1 |
| 2 | HELIUM | He | 1s2 | 2 |
| 3 | LITHIUM | Li | 1s2 2s1 | 2,1 |
| 4 | BERYLLIUM | Be | 1s2 2s2 | 2,2 |
| 5 | BORON | B | 1s2 2s1 2p1 | 2,3 |
| 6 | CARBON | C | 1s2 2s1 2p2 | 2,4 |
| 7 | NITROGEN | N | 1s2 2s1 2p3 | 2,5 |
| 8 | OXYGEN | O | 1s2 2s1 2p4 | 2,6 |
| 9 | FLUORINE | F | 1s2 2s1 2p5 | 2,7 |
| 10 | NEON | Ne | 1s2 2s1 2p6 | 2,8 |
| 11 | SODIUM | Na | 1s2 2s1 2p6 3s1 | 2,8,1 |
| 12 | MAGNESIUM | Mg | 1s2 2s1 2p6 3s2 | 2,8,2 |
| 13 | ALUMINIUM | Al | 1s2 2s1 2p6 3s2 3p1 | 2,8,3 |
| 14 | SILICON | Si | 1s2 2s1 2p6 3s2 3p2 | 2,8,4 |
| 15 | PHOSPHORUS | P | 1s2 2s1 2p6 3s2 3p3 | 2,8,5 |
| 16 | SULPHUR | S | 1s2 2s1 2p6 3s2 3p4 | 2,8,6 |
| 17 | CHLORINE | Cl | 1s2 2s1 2p6 3s2 3p5 | 2,8,7 |
| 18 | ARGON | Ar | 1s2 2s1 2p6 3s2 3p6 | 2,8,8 |
| 19 | POTASSIUM | K | 1s2 2s1 2p6 3s2 3p6 4s1 | 2,8,8,1 |
| 20 | CALCIUM | Ca | 1s2 2s1 2p6 3s2 3p6 4s2 | 2,8,8,2 |
| 21 | SCANDIUM | Sc | 1s2 2s1 2p6 3s2 3p6 3d1 4s2 | 2,8,9,2 |
| 22 | TITANIUM | Ti | 1s2 2s1 2p6 3s2 3p6 3d2 4s2 | 2,8,10,2 |
| 23 | VANADIUM | V | 1s2 2s1 2p6 3s2 3p6 3d3 4s2 | 2,8,11,2 |
| 24 | CHROMIUM | Cr | 1s2 2s1 2p6 3s2 3p6 3d5 4s1 | 2,8,13,1 |
| 25 | MANGANESE | Mn | 1s2 2s1 2p6 3s2 3p6 3d5 4s2 | 2,8,13,2 |
| 26 | IRON | Fe | 1s2 2s1 2p6 3s2 3p6 3d6 4s2 | 2,8,14,2 |
| 27 | COBALT | Co | 1s2 2s1 2p6 3s2 3p63d7 4s2 | 2,8,15,2 |
| 28 | NICKEL | Ni | 1s2 2s1 2p6 3s2 3p6 3d8 4s2 | 2,8,16,2 |
| 29 | COPPER | Cu | 1s2 2s1 2p6 3s2 3p6 3d10 4s1 | 2,8,18,1 |
| 30 | ZINC | Zn | 1s2 2s1 2p6 3s2 3p6 3d10 4s2 | 2,8,18,2 |
The above table gives electronic configuration of first 20 elements
Electronic configuration of iron
Iron is a one-of-a-kind element that exists both outside and inside us. Iron has 8 valence electrons and an electron configuration of 1s2 2s1 2p6 3s2 3p6 3d6 4s2, which means it has
-
K shell – 2 electrons,
-
L shell – 8 electrons,
-
M shell – 14 electrons, and
-
N shell – 2 electrons.
Iron is a silvery white metal that is ductile and malleable under normal conditions. Iron is a medium-activity metal that extracts hydrogen from water solutions of strong acids like HCl and sulphuric acid, resulting in the formation of iron salts.
What Function Does Electronic Configuration of Elements Play?
The chemical characteristics of elements are largely determined by their electronic arrangement. Despite their small size, electrons are responsible for determining the nature of the elements. They determine the valency, ionisation potential, ionisation enthalpy, chemical bonding, and practically all other chemical properties of the element. When an element lacks an electron, it is classified as an electron acceptor, and when it has an excess electron, it is classified as an electron giver. As a result, electrical configuration, like the electron, is a deciding factor.
Also read -
-
NCERT Solutions for Class 11 Chemistry
-
NCERT Solutions for Class 12 Chemistry
-
NCERT Solutions for All Subjects
Frequently Asked Question (FAQs) - Electronic Configuration of First 30 Elements - Overview, Structure, Properties & Uses
Question: Why is chromium's electrical configuration unique?
Answer:
-
There are two key reasons for this: the 3d orbital has a lower energy, and lowering repulsions in the 4s orbital by shifting one of the 4s electrons to a close-lying 3d orbital reduces the chromium ground-state energy. The 3d- orbital as well as 4s- orbital have almost equal energy levels and chromium atom has 4- electrons in the d- orbital. So to attain stability it has to be half-filled.
Question: Is removing an electron from sodium or aluminium easier?
Answer:
It does not take much energy to extract one electron from a sodium atom and generate a Na+ ion with a filled-shell electron configuration. The second ionisation energy of aluminium is higher than the first, and the third is much higher.
Question: What is beryllium electronic configuration?
Answer:
The electronic configuration of beryllium is 1s2 2s2.
Question: What is the atomic number of titanium?
Answer:
The atomic number of Titanium (Ti) is 22 with an electronic configuration of 1s2 2s1 2p6 3s2 3p6 3d2 4s2.
Question: What is copper's electronic configuration?
Answer:
Copper has the electrical configuration [Ar] 3d104s1. Due to the narrow energy gap between the 3d and 4s orbitals, this structure violates the Aufbau principle. The fully filled d-orbital arrangement is more stable than the partially filled one.
Electronic Configuration of First 30 Elements
Chemistry 1 to 30 Elements Electronic Configuration
Source: https://school.careers360.com/chemistry/electronic-configuration-of-first-30-elements-topic-pge

0 Response to "Chemistry 1 to 30 Elements Electronic Configuration"
Post a Comment